In one of our University Laboratory Studies, we demonstrated that <33™ Technology with Hydration Formula increases the integrity of cell membranes and health of frog oocytes (unfertilized egg cells).
Cell Health
Using patch-clamp measurements of cell membrane potential, we demonstrated that the membranes of cells exposed to the <33 Technology increased their electrical potential over five consecutive days compared to cells exposed to a ‘sham’ condition. In fact, the untreated cells showed a reduced membrane potential over this period suggesting compromised membrane integrity.
Background.
Prof. Dr. Branda and his team designed and conducted studies to help identify what are the discernible differences in cell health when they are incubated with growth media conditioned with <33 Technology Hydration Formula compared to media that is not conditioned. We used patch clamp electrodes to measure ion regulation across cell membranes. This method is highly controlled, repeatable and recognized by the scientific community at large. Because the cell membrane separates the intracellular ions (K+, Na+, Ca2+, Cl–, etc.) from the extracellular ions and because these two concentrations are different, an electrochemical gradient is established. The difference in potential between the inside and outside of the cell can be measured using the patch clamp technique. As the health of the cell decreases, the cell’s ability to regulate this concentration gradient is impaired. Some ions may leak across the membrane due to a poorer quality of the phospholipid bilayer. Since the technique measures a difference in potential between the outside and inside of the cell, 0 mV would represent a situation where there is no difference in potential between the inside and outside compartments. This can be a result of a faulty or “leaky” membrane. The larger the potential, the greater is the cell’s ability to regulate ion transport.
Frog oocytes (unfertilized egg cells) were chosen as the model cells to examine the effect on the <33 conditioning on cell health. These specific cells were chosen because they are relatively large in size (as much as 1 mm in diameter), are robust and their health easy to monitor using patch clamp techniques to examine cell membrane potentials. The rationale for the experiments was as follows:
If water conditioned with the <33 shows a higher tendency to solvate dissolved ions, this solvating ability of <33 treated water may be observable as a greater ion mobility across cell membranes, or at least, a difference in how the cell membranes regulate ion transport. The implications of this phenomenon have relevancy to many life sciences applications.
Cells incubated in media conditioned using <33 plates showed significantly more negative membrane potentials, indicating a tighter regulation of ion movement across the cell membranes compared to cells incubated in untreated media.
Materials and Methods.
Oocyte preparation. Xenopus laevis oocytes (Figure 1) were isolated from frogs during recoverable surgical procedures. Oocytes were isolated and defolliculated via a combination of collagenase treatment (1 h in 1 mg/ml collagenase) and manual defolliculation. After treatment, oocytes were incubated in SOS+ media (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, 5 HEPES, 5% horse serum, 2.5 sodium pyruvate, 100 mg/L gentamicin sulfate, pH 7.4). A total of 32 oocytes were divided into 2 groups:
- 16 were incubated in SOS+ media (‘control’ group)
- 16 were incubated in SOS+ media then placed on <33 Technology plates (‘<33 conditioned’ group).
The two groups of oocytes were stored in separated incubators at 19°C so as not to cross-contaminate the conditioning. The <33 plates remained under the ‘<33 conditioned’ group for the duration of the experiment. The solutions were switched once a day (both the untreated ‘control’ and ‘card conditioned’ groups receiving fresh SOS+ media). At no time during the preparation did the cells in each group come into close proximity with each other.

Figure 1. Microscope image showing typical Xenopus laevis oocytes showing the light and dark hemispheres typical for these types of egg cells.
Data Acquisition and Analysis.
Membrane potentials were recorded for oocytes using two-electrode voltage clamp (Figure 2) while the cells were bathed in ND96 solution (in mM: 96 NaCl, 3 KCl, 1 MgCl2, 0.5 CaCl2, and 5 HEPES, pH 7.4). Microelectrodes had a resistance of 0.2–2.0 MΩ when filled with 3 M KCl. Experiments were performed at 20–22°C. At no time during the data acquisition did the cells in each group come into close proximity with each other. After one day of incubation, two cells from each group were tested as a preliminary inspection to determine if any differences in membrane potentials were present. This procedure was repeated as indicated in the raw data (see the Table at the end of this report) until day 3, at which time the 4 cells from each group were tested due to the fact that the first two cells of the ‘<33 conditioned’ and ‘control’ groups showed significantly differences. Further experimentation with the remaining 2 cells negated this. No testing occurred on day 4. The remaining 8 cells from each group were tested on day 5. Mean membrane potentials were analyzed against each group using a One-way ANOVA test. A significant difference was considered to be a p value less than 0.05 (p<0.05)
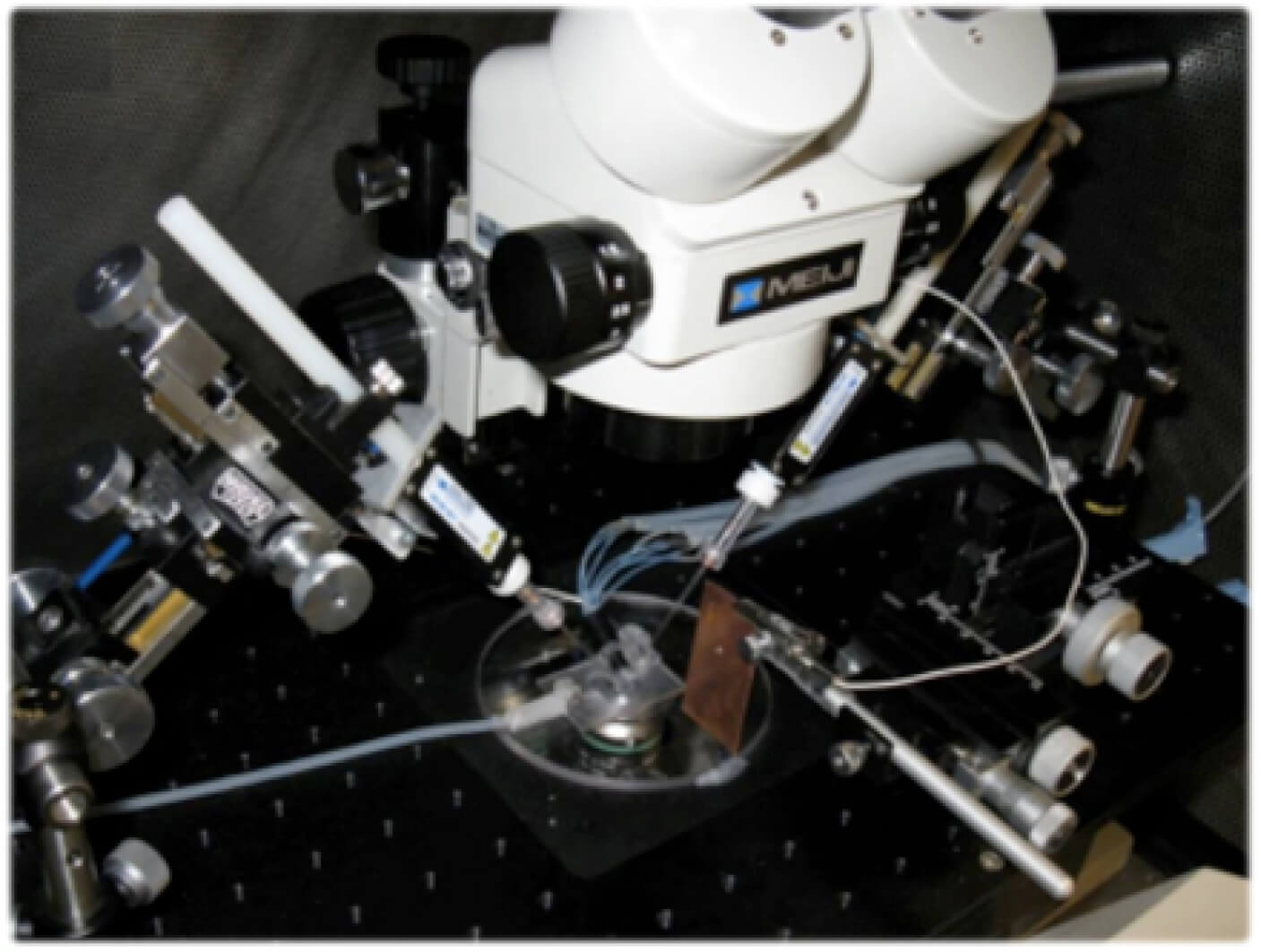
Figure 2. Typical experimental set-up to measure membrane potentials using patch clamp electrodes.
Results.
The ‘iC-iT conditioned’ group of oocytes had membrane potentials that were significantly different from the ‘control’ group. After 5 days of incubation, the mean membrane potential of cells in the ‘control’ and ‘<33 conditioned’ groups were –26.4 ± 3.7 mV and –42.6 ± 2.6 mV, respectively (Figure 3).
A One-way ANOVA test revealed that the mean membrane potential of the ‘<33 conditioned’ group was significantly different from the mean membrane potentials of the ‘control’ group (p < 0.05). There was no observable difference in the physical appearance of oocytes of each group (see the Table at the end of this report).
Figure 3. Membrane potentials for oocytes over 5 days as they are incubated in untreated growth media and media conditioned with a <33 Technology plate.
Discussion.
After treatment with the <33 Technology for 5 consecutive days, there was a significant difference in the membrane potentials of the oocytes when compared with oocytes in the ‘control’ group.
Oocyte membrane potentials can be used to describe cell health – a more negative membrane potential correlates with a healthier membrane and a tighter regulation of ion movement across the membrane. Because the magnitude of membrane potentials are governed by the concentration of charged ions on either side of the cell’s membrane we can conclude that after treatment with the <33, there exists a difference in the separation of charge across the membrane. This may be attributed to a difference in membrane permeability. The membranes of the oocytes in the ‘<33 conditioned’ group may be “healthier” or less porous. Either phenomenon could set up a greater separation of ions on either side of the membrane, presumably the more positive ions being located on the outside of the cell resulting in a more negative environment inside the cell. The ‘control’ group in comparison to the ‘<33 conditioned’ group may have more permeant membranes. This may occur if the quality of the membrane is compromised. The more porous membrane loses its ability to effectively contain intracellular contents and as a result, ions from inside the cell may be able to leak through to the extracellular solution. This sets up less separation in ion concentration on either side of the membrane.
Dr. Neil Branda
Professor of Chemistry and Canada Research Chair in Materials Sciences
Burnaby